Why the genetic-testing revolution left some people behind — and what to do about it


Evolutionary geneticist Mary-Claire King did not anticipate the impact of her discovery.Credit: Rina Castelnuovo/New York Times/Redux/eyevine
When Mary-Claire King embarked on a painstaking 17-year-long hunt for a gene linked to breast cancer, she had no inkling that its discovery would be saving lives some three decades later.
King, an evolutionary geneticist, was trying to solve the mystery of why breast cancer was common in some families. This was during the 1970s, decades before the first human genome was sequenced. In the absence of modern tools such as PCR tests, sequencing and mapping genes took a heroic effort. Cancer researchers at the time were mostly studying tumour-causing viruses, but several individuals having the disease across generations of the same family suggested that considerable danger could lurk in the human genome, too.
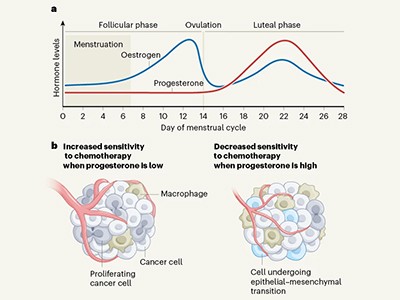
What is the best time of the month to treat breast cancer?
“The worldwide impact of something like this just never crossed my mind,” says King, who is at the University of Washington in Seattle. “I was absolutely gobsmacked.” King named the gene BRCA1. Since then, it has become clear that mutations in BRCA1 are responsible for about 35% of hereditary breast cancers, and that genetic variants of it and a related gene called BRCA2 are also linked to ovarian, prostate and pancreatic cancers. Drugs have been developed that target cancers with these variants, and genetic tests are available to identify people who are at risk.
But looking back on the impact of BRCA1’s discovery also highlights how far there still is to go. Too few people have access to genetic tests and, even when they do, they have few options to reduce their risk of cancer. Researchers must advocate for and study ways to improve access and to expand the cancer-prevention options available to people who carry BRCA1 and BRCA2 mutations.
Huge leap in breast cancer survival rat
BRCA1 encodes a protein that is important for repairing damaged DNA. Although King identified the BRCA1 gene and pinpointed its location1 in 1990, the team that first sequenced it in 1994 included researchers at the precision-medicine firm Myriad Genetics in Salt Lake City, Utah2. Myriad promptly applied for patents on the gene and used this intellectual property to prevent competitors from developing tests for cancer-associated BRCA1 mutations. The high price tag of Myriad’s genetic tests kept them out of many people’s reach, until a landmark US Supreme Court decision in June 2013 found that such gene patents were invalid.
Following the court’s decision, test prices in the United States plummeted from around US$3,800 to $250, as other providers surged into the field. Yet, testing remains limited, despite studies3 finding that expanding BRCA1 and BRCA2 testing to all women could be cost-effective, particularly for those screened between the ages of 20 and 35. There are several reasons for this, including limited health-care access and concerns about privacy. Lack of awareness among primary-care physicians about genetic testing and conflicting guidelines from professional organizations about who should be tested contribute, too. For now, however, even in places where testing is an option, it is often made available only to those at high risk of carrying a cancer-associated form of BRCA1, including people with a high rate of cancer in their family (see ‘Testing times’).
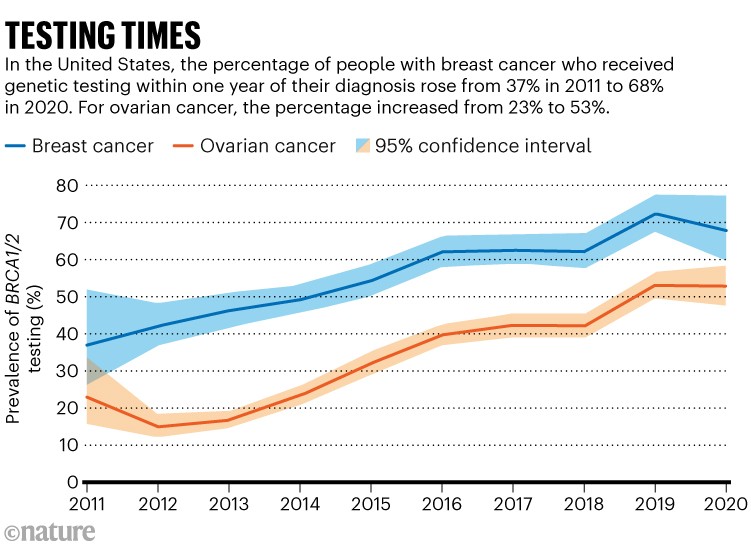
Source: Ref. 4
Many who are eligible do not get tested for BRCA1 and BRCA2 mutations: one US study4 found that only about 35% of eligible individuals with ovarian cancer and 56% of eligible people with breast cancer had been tested. Other problems limit the tests’ practical benefits, too. Reports provided to physicians and people with cancer are often unnecessarily complicated, because they list not only mutations known to increase risk, but also any other unusual DNA sequences in the genes — even if their relevance is unknown. Many tests also provide data on genes unrelated to cancer, launching fresh medical odysseys for people already dealing with a cancer diagnosis. When King accompanied a friend diagnosed with breast cancer to a clinical appointment, the attending doctor waved off suggestions for BRCA1 testing. “The difficulty with genetic tests,” they said, “is that they simply beget more tests.”
This scientist treated her own cancer with viruses she grew in the lab
Simplifying tests and equipping medical staff with the knowledge to interpret the results could improve uptake. People who learn that they carry worrisome BRCA1 mutations need better options to either prevent cancer from developing or intercept it at an early stage. This is particularly crucial for reducing the risk of ovarian cancer and aiding its early detection. Whereas mammograms can detect some breast tumours early, there is no equivalent test for ovarian cancer, which is often diagnosed at late stages. At present, cancer detection and prevention are typically achieved by careful monitoring or, in some cases, surgery to remove the breasts and ovaries. “When I see a 25-year-old woman newly found to have a BRCA1 mutation, I’m mostly having the same conversations now that I did long ago for her options for risk reduction,” says Susan Domchek, a breast-cancer specialist at the University of Pennsylvania Perelman School of Medicine in Philadelphia. “We have a lot of work to do.”
To improve on this, researchers must develop better means of detecting cancers early, and learn more about the biology of early tumours and why some will go on to become malignant whereas others do not. They must also investigate ways to treat people at earlier stages — an effort that will require learning more about early cancers’ biological hallmarks. By contrast, most treatments are first developed for and tested in people who have advanced disease.
By filling the gaps on testing and giving people with harmful mutations better ways to reduce their risk, BRCA1 and BRCA2 testing could become a model for how genetic tests for other cancer risk factors should be implemented. Then, King’s crucial discovery will save even more lives.
Source link